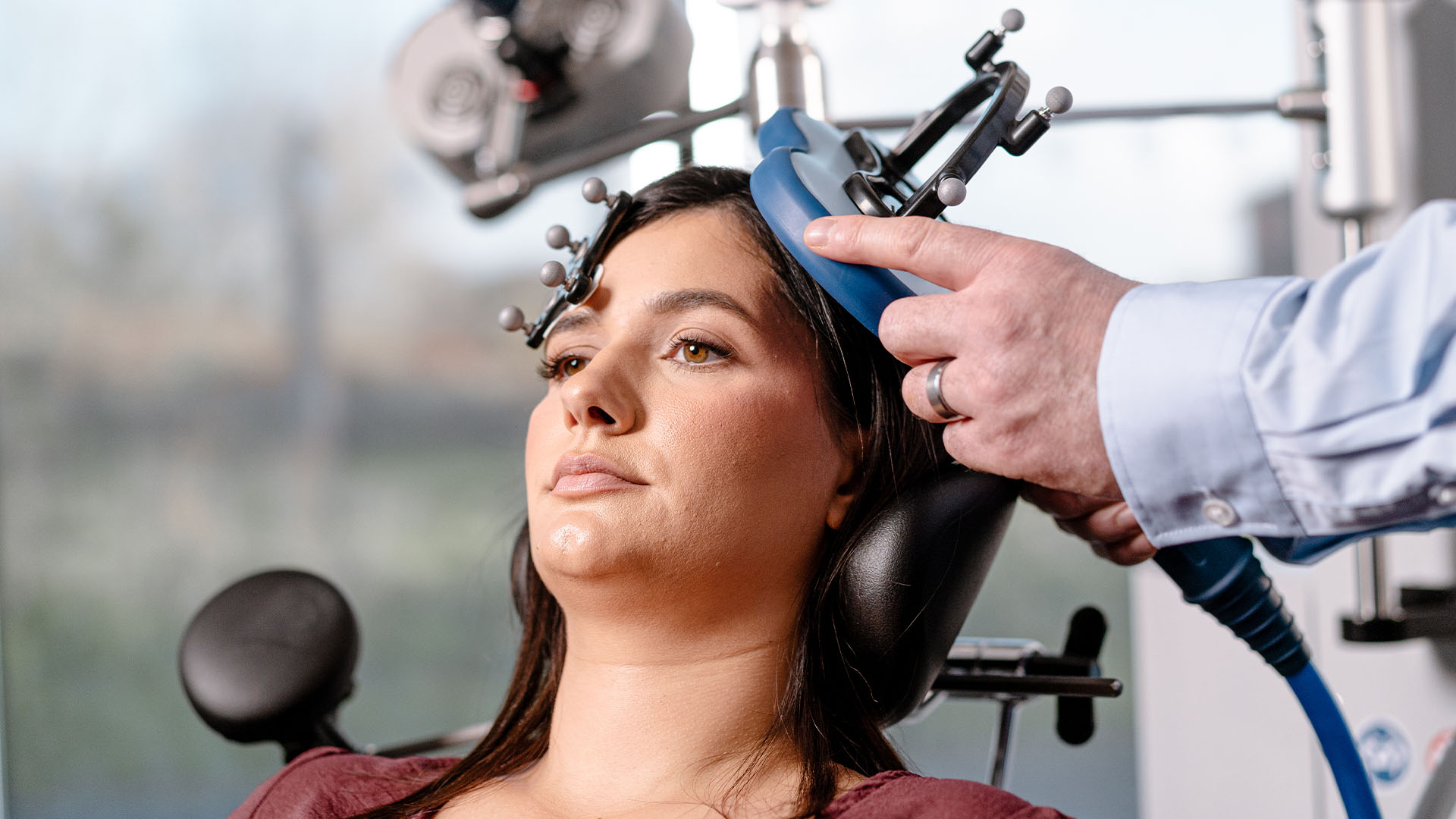
What is Transcranial Magnetic Stimulation – TMS
Transcranial magnetic stimulation (TMS) is:
- An FDA approved, non-invasive outpatient procedure.
- No need for anesthesia or a surgical procedure.
- A TMS coil is positioned on the patient’s scalp and the TMS stimulator delivers painless, magnetic pulses to the targeted brain region.
- TMS therapy involves daily sessions over several weeks, enabling patients to quickly return to normal activities after treatment.
- Comfortable and stable treatment experience.
- Patients can have personalized treatment plans, tailored to their medical needs.
- Ongoing research is exploring the potential and efficacy of TMS for the treatment of other medical conditions, e.g. Alzheimer’s disease, Chronic pain, Parkinsons disease, Schizophrenia and more.
Learn More About TMS
The benefits of Transcranial Magnetic Stimulation (TMS) include:
- Non-Invasive procedure: TMS is a non-invasive procedure, meaning it does not require any form of surgery or anesthesia.
- Minimal Side Effects. Outpatient Procedure: TMS is typically performed on an outpatient basis, allowing patients to return home immediately after treatment sessions. This convenience reduces disruptions to daily life and eliminates the need for hospitalization.
- Targeted Treatment: precisely target areas of the neural tissue. This targeted approach may result in more effective treatment.
- Positive Results: Many patients experience improvement in symptoms within a few days of starting TMS therapy, unlike some medications that may take weeks to become effective.
- Long-lasting Effects: Studies suggest the benefits of TMS therapy can be felt beyond the treatment period, providing lasting relief from symptoms.
Magstim is supported by industry-leading hospitals and clinics throughout the world. With demonstrable clinical heritage and extensive contribution to the field of neuroscience is evident with over 15,000 papers published, showcasing its significant impact on research and clinical practice. This vast body of literature underscores the trust and reliance researchers place in Magstim’s products and technologies. It highlights Magstim’s commitment to advancing neuroscience and improving patient care worldwide.
In the 1980s, the initial trials exploring Transcranial Magnetic Stimulation (TMS) took place utilizing Magstim’s TMS stimulator. Since then TMS technologies have significantly advanced with more patients being treated using TMS therapies.
Transcranial Magnetic Stimulation (TMS) therapy was initially cleared by the USA Food and Drug Administration (FDA) in 2008 as a treatment for adults with Major depression disorder (MDD) who have failed to respond to at least one antidepressant.
Since 2010, TMS therapy has been recommended by the American Psychiatric Association for the treatment of major depressive disorder and is covered by most major insurers including Medicare. Additionally more states are covering TMS therapy under their Medicaid policy.
Ongoing progress in TMS technology, including enhancements in coil design, stimulation protocols, and targeting methods, has strengthened its therapeutic effectiveness and expanded the range of applications.
TMS is widely acknowledged as a safe, efficient treatment solution for a diverse range of neurological and psychiatric conditions. Research continues globally using the Magstim TMS brand to explore its full range of benefits.
Contact your physician or check out our list of TMS Providers to find a physician near you who offers TMS treatment.







