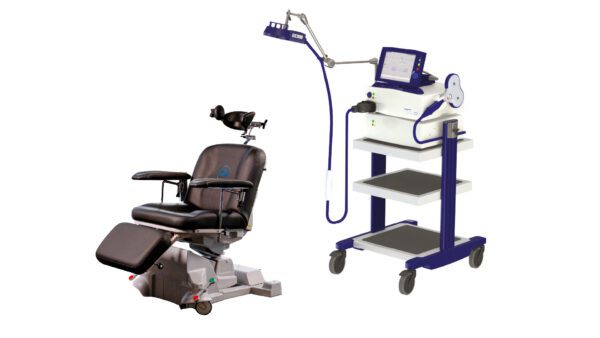
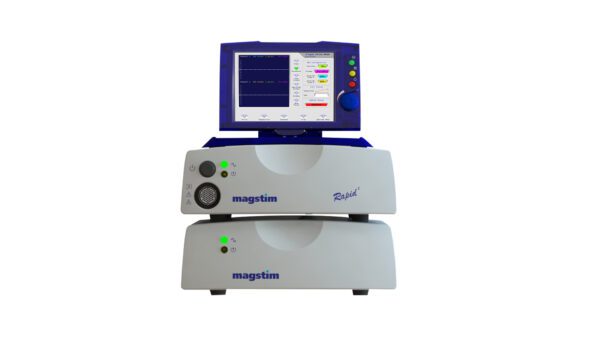
Horizon 3.0 with StimGuide Pro
Building on the foundations of the Horizon 3.0, now with StimGuide Pro.
Elevate patient care and advance your practice with the Magstim Horizon 3.0. The Horizon range has been designed with intuitive, user-friendly interfaces and treatment protocols, which provide clinicians and researchers with the flexibility to tailor stimulation parameters to individual patient needs, ensuring optimal treatment outcomes. Available with/without navigation. Horizon 3.0 is FDA cleared for clinical treatment of MDD, OCD, and decreasing Anxious Depression symptoms*. For a full list of indications, please click here.
Features
Built in Navigation
Introducing StimGuide Pro, where repeatability and accuracy matter the most.
Data Analytics
Powerful data analytics to monitor patient treatment progress
Clinical Workflow
Preset clinical workflows for ease of use/delivering reliable treatments
No Pulse Decay
Correct and consistent dosage amounts with no pulse decay
Specifications
The best there is to offer from a clinical navigation system, where repeatability and accuracy matter the most. The use of StimGuide Pro is designed to enhance the accuracy of coil placement during TMS sessions. This reduces the risk of off-target stimulation and ensures that the magnetic pulses are delivered to the intended brain regions, optimizing the therapeutic effects.
This is particularly crucial in TMS, where the efficacy of the treatment often depends on accurate and targeted stimulation of neural circuits associated with the patient’s condition.
Clinicians can have greater confidence that the delivered TMS pulses are reaching the intended targets, leading to more consistent and predictable therapeutic results.
System features:
• Quality metrics to ensure all pulses are delivered.
The software is designed to display the stimulator control, EMG and Navigation all within one application, condensing the system down to one touchscreen monitor.
Features include:
• Visual approximation of all 10/20 electrode positions.
• Four points of anatomical registration for improved accuracy of the size of the patient’s head.
• Anatomical Landmark registration check.
(Visibility of the pointer tool on screen after registering landmarks).
• Advanced on-screen visualization of the Pointer tool (full scale) and Coils (1/3 Scale).
• Gimbal navigation aid for rotation and tilt movements.
• Navigation by coordinate input.
New Tracking Camera with an increased viewing volume.
• Typical positional accuracy of the Navigation Camera: ≤ ± 0.2 mm.
• Typical rotational accuracy of the Navigation Camera: ≤ ± 0.1°.
All new Navigation Tracker Tools, featuring four points of reference each, allow for more accurate positioning of the coils.
Remote Pointer Tool allowing complete freedom for anatomical landmark registrations.
Fully integrated with Magstim Connect and Analytics, allowing the navigation data to be saved and recalled from any Horizon 3.0 StimGuide Pro within the installation’s organization. See our section on Magstim Connect for more information.
- State-of-the-art technology backed by Magstim’s legacy of innovation.
- Streamlined treatment with an optimized software workflow.
- Enhanced patient data management and connectivity through Magstim Connect.
- Comfortable and stable treatment experience with the electronically actuated Treatment Chair.
- Precise and personalized treatment plans based on Motor Threshold assessment.
- Cutting-edge technology for improved treatment outcomes and overall well-being.
- A more comfortable pulse width. Shorter pulse widths can cause increased discomfort.
Electronically actuated devices are designed to allow movement for optimal patient comfort and stability during treatment. A specially designed headrest minimizes patient movement without compromising patient comfort, ensuring the head remains securely in place for precise TMS application.
Designed for motor threshold assessment. Controls located on the coil for ease of use.
Horizon 3.0 is FDA-cleared for clinical treatment of MDD, OCD, and decreasing Anxious Depression symptoms*.
* The FDA has previously cleared the Magstim Horizon 3.0 TMS Therapy System for the treatment of depressive episodes for decreasing anxiety symptoms for those who may exhibit comorbid anxiety symptoms in adult patients suffering from Major Depressive Disorder (MDD) – also known as Anxious Depression.
To view the full range of Magstim’s indications, please click here.
Contact Us
Contact us