Magstim – New FDA Clearance for TMS Treatment in U.S. Adolescent Patients
Exciting News in Mental Health Treatment
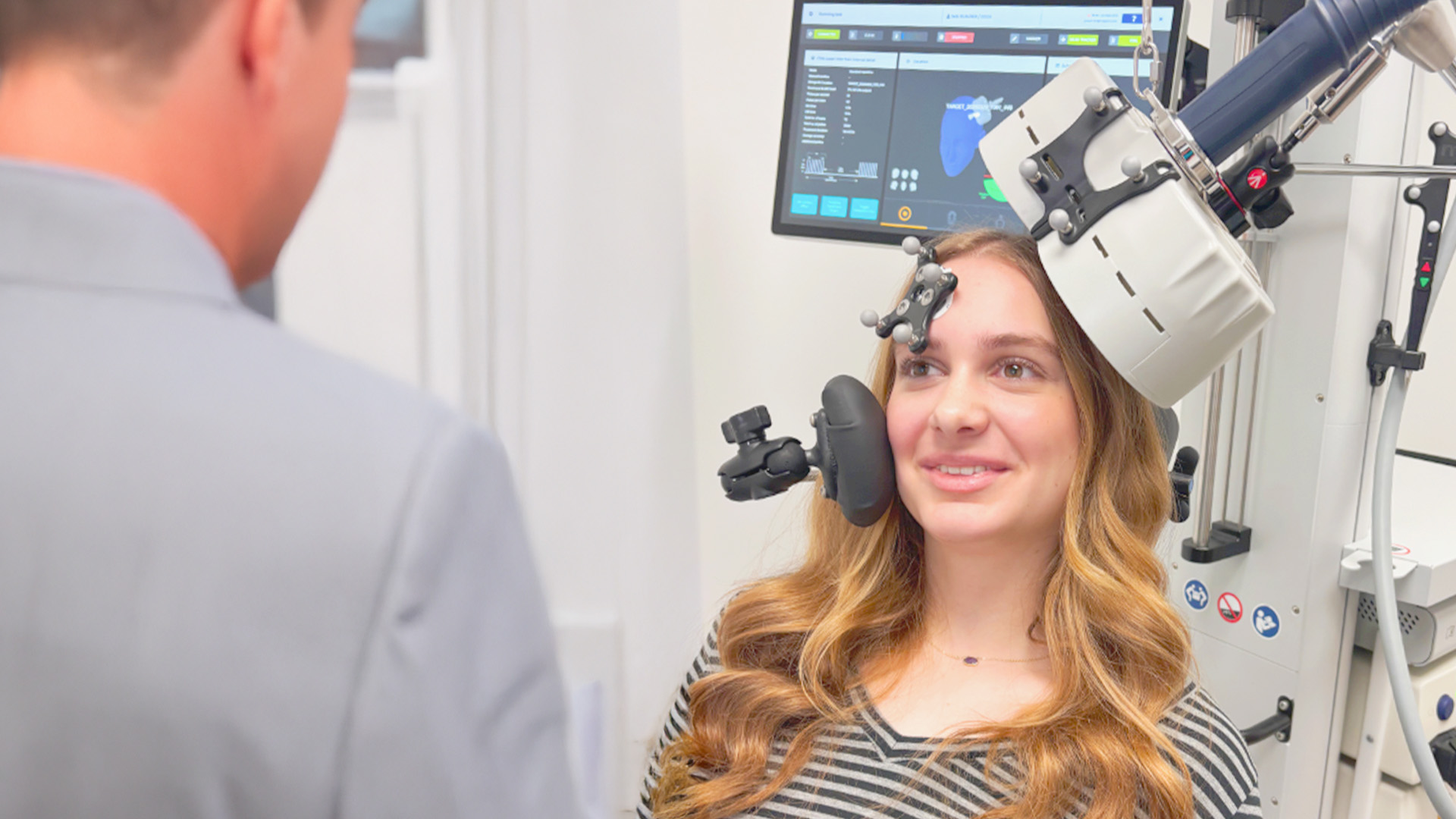
Magstim Horizon Inspire and 3.0 Transcranial Magnetic Stimulation Systems have officially received FDA clearance for the treatment of Major Depressive Disorder (MDD) in adolescent patients, ages 15-21. This marks a significant milestone in mental health care, opening new doors for more effective, non-invasive treatment options tailored specifically for younger patients battling depression.
A Groundbreaking Advancement in Adolescent Care
The FDA clearance of the Horizon Inspire and 3.0 Transcranial Magnetic Stimulation systems represents a powerful step forward in offering hope and better outcomes for adolescents struggling with MDD. These advanced systems leverage leading Transcranial Magnetic Stimulation technology to deliver precise and effective treatment in a safe, non-invasive manner.
By providing a solution for adolescent patients, Magstim is helping to address the critical need for innovative mental health treatments during one of the most formative periods of life. Transcranial Magnetic Stimulation offers a unique alternative to traditional therapies, providing a personalized approach that can significantly improve the lives of young individuals experiencing depression.
Transforming the Future of Mental Health Treatment
With the clearance of these FDA-approved Horizon Transcranial Magnetic Stimulation systems, we are excited to be part of the movement to transform the landscape of mental health treatment for younger generations. This advancement is a crucial step in ensuring that adolescents have access to the best possible care as they navigate mental health challenges.
If you’re interested in learning more about how the Magstim Horizon Transcranial Magnetic Stimulation Systems can support adolescent MDD treatment, we invite you to book a free virtual demonstration today!
Click here to schedule your virtual demo: https://www.magstim.com/contact-us/
Note:
For Adults: Horizon 3.0 Transcranial Magnetic Stimulation Therapy Systems are indicated for the treatment of Major Depressive Disorder (MDD) in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode, as well as an adjunct for the treatment of adult patients suffering from Obsessive-Compulsive Disorder (OCD). For Adolescents: Horizon 3.0 Transcranial Magnetic Stimulation Therapy Systems are indicated as an adjunct for the treatment of MDD in adolescent patients (age 15-21).


